Hi-tech hopes in cancer fight
Technology brings better treatment for patients, lower costs for health care
By Greg BaskySince the 1970s, medical physicists -- the scientists behind many advances in cancer fighting technology and treatment planning -- have pursued two goals: To more precisely visualize the cancer tumours that doctors are trying to treat, and to enable them to more precisely treat those tumours.
A breakthrough pioneered by USask graduate Brad Murray (BE’85) promises to deliver unprecedented levels of precision on both fronts.
The new treatment system, branded the Aurora-RT, combines the real-time, high definition imaging capabilities of an MRI (magnetic resonance imager) with the cancer-blasting radiation delivery of a linear accelerator (Linac).
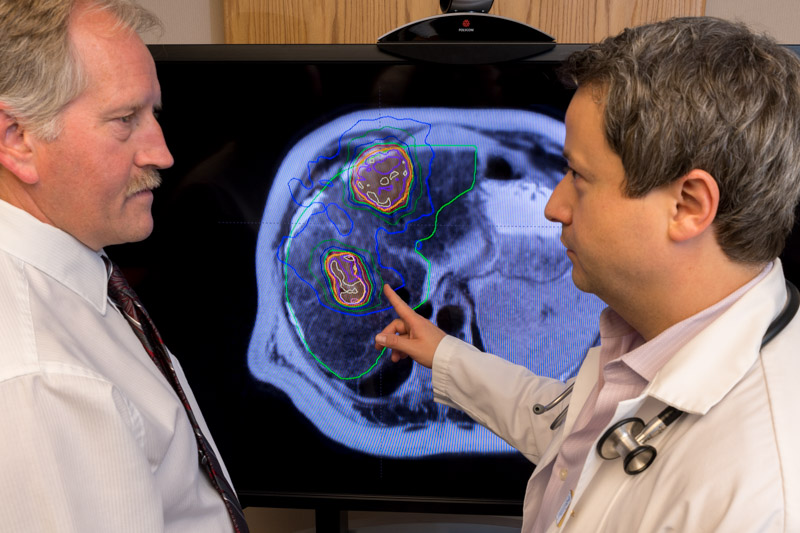
A major limitation of the current gold standard in image guided radiotherapy, tomotherapy, is that because its imaging component, computerized tomography (CT), uses X-rays, the image quality is not as good as an MRI and there is a lag between image capture and delivery of the beam of radiation.
During that gap, a patient’s breathing can cause organs or the tumour itself to shift; tumours in the lung and liver are especially susceptible.
The machine, however, cannot detect this movement, and as a result more surrounding healthy tissue gets hit with radiation. Because up to half of cancer treatment sites can be affected by movement, as many as half of all cancer patients could potentially benefit from this new technology, according to Dr. Thomas “Rock” Mackie, the inventor of tomotherapy.
The new system developed by Murray and his partner Dr. Gino Fallone (University of Alberta) uses MRI’s real-time imaging, so it can adjust or stop the radiation if it picks up any movement in a cancer tumour or organs. Delivering too much radiation to healthy surrounding tissue or organs can be worse than cancer itself.
“We can increase the likelihood of cure and minimize the likelihood of causing significant side effects. Those side effects typically come when you’re hitting healthy tissue,” said Murray who was until January 2020 a clinical professor in the U of A’s Department of Oncology, and senior medical physicist at the Cross Cancer Institute. He is now chief technical officer with MagnetTx Oncology Solutions, the company behind the Aurora-RT.
This newfound precision also holds considerable potential to reduce the costs of treatment, great news for financially strapped health-care systems everywhere.
Early results suggest that, for certain cancers -- prostate, for example -- the Linac-MR can substantially reduce the number of treatments required. According to Murray, in certain groups of prostate cancer patients they’ve been able to achieve in just five treatments the same results that previously took 39 sessions.
“The standard for radiotherapy has been the same for pretty much -- I hate to say it -- but probably close to a hundred years. We’ve typically treated cancer with a small amount of radiation every day or every few days. The Linac-MR is going to bring more precision and allow us to treat in much higher doses per day, but many fewer days.”
Engineering marvel: Doing the impossible
The Aurora-RT successfully marries in one machine two technologies previously thought incompatible. Until now, the Linac’s massive radio frequency power source would distort images captured by an MRI. And the strong magnetic field that an MRI machine produces bends the beam created by the Linac in such a way that no radiation would be produced.
In fact, installation instructions for Linacs call for them to be sited at least 10 metres away from an MR; similarly MR installation instructions advise locating the unit a minimum of 10 metres away from a Linac.
Murray and Fallone wondered: Could we mount a linear accelerator on an MRI so it was aligned with the main magnetic field and rotate both the linear accelerator and the MR magnet -- together, in parallel -- around the patient, so that the MR wouldn’t “see” the Linac?
“That, as they say, is the $64-million question,” said Murray. Their breakthrough came when the pair decided to swap in a special type of magnet, called a bi-planar magnet.
In December 2008, Murray and Fallone built their first proof of principle machine that allowed them to irradiate an object and take an MR image at the same time.
“When we did that, we were the first in the world to irradiate with a Linac mounted directly onto an MR and to be able to image while we were irradiating,” said Murray. “That was a fairly significant moment. It was a huge step forward.”
Fallone credits Murray’s “detailed thinking and being resolute in resolving the engineering issues” that arose throughout development for helping bring the Aurora-RT to life. Brad is a calm thinker and practical implementor, according to Fallone.
“If there’s an issue, he goes straight to resolving it, which is a sign of a good physicist. He tries to fix the problem.”
The innovation is the latest in a string of major advances in radiation treatment, or radiotherapy, that trace their roots back to the University of Saskatchewan.
In the late 1940s, medical physicist Harold Johns -- recognized as the grandfather of that field in Canada -- did his pioneering research into the use of Cobalt-60 in radiation treatment of cancer here at USask. Sylvia Fedoruk, who did both her BA and MA (Physics) at USask, was also involved in the development of the Cobalt-60 and was chief medical physicist at the Saskatoon Cancer Clinic. And Thomas “Rock” Mackie, who developed the first radiotherapy machine with a built-in CT scanner (tomotherapy), did his undergraduate degree in physics at USask.
Is the Linac-MR system yet another milestone in the field of radiation therapy?
“Absolutely,” says Mackie. Murray is flattered to be included with those three icons of medical physics. “My career cannot match their stature, but I do find it interesting how prominent the U of S is in the major developments of radiotherapy.”
A meeting in late 1987 set Murray down the path to becoming a medical physicist. Over coffee, Dr. Bill Reid, the head of medical physics at the Saskatoon Cancer Centre at the time, and the centre’s senior physicist Dr. Alistair Baillie were selling Murray on the merits of a career in their field. That conversation led to a job offer, to commission new equipment that was being installed at the centre.
Baillie recommended Brad pursue his Masters in medical physics at the University of Alberta, regarded as one of the top programs in the country. Under thesis supervisor Dr. John Scrimger, Murray evaluated the accuracy of a new treatment planning algorithm designed to simulate dose delivery. His work was based at the Cross Cancer Institute, which essentially functioned as the home of the U of A’s medical physics department.
“It gave me a great environment for learning both the clinical and academic side of that program.”
Sizing up the competition
The Aurora-RT is up against a couple of competitors that have capitalized on the MR-Linac concept pioneered by Murray and Fallone. However, the Alberta team’s product is well positioned to succeed, says Mackie, because it hits the sweet spot between the machines being produced by European multinational Elekta and American startup ViewRay.
The Aurora-RT has a stronger magnetic field than the unit from ViewRay, so it produces clearer images, with less noise. And the magnet strength in Elekta’s product is too strong, which tends to distort the radiation beam produced by the linear accelerator. “I think they’re a better compromise between their two commercial competitors.”
In the MagnetTx machine the beam generated by the Linac is aligned with the magnet, not bent by it. “In fact, it (Aurora-RT) focuses it a little, so that the beam is actually sharper,” says Mackie.

Another competitive advantage, according to Murray, is that their bi-planar magnet leaves more room for the patient opening, so doctors can move patients up to 25 centimetres laterally, to better align the radiation beam with the tumour. The Elekta and ViewRay machines have almost no room to move a patient -- and tumour -- away from centre, which limits their ability to attack cancers in certain sites -- especially breast and lungs.
One of the biggest challenges the pair has faced is attracting funding. Developing radiotherapy systems isn’t cheap.“Raising money was always difficult,” said Murray. The pair’s original cash injection was a $500,000 grant from the Alberta Cancer Foundation (ACP). Murray credits Dr. Tony Fields, then director of the Cross Cancer Institute, for convincing the Cancer Foundation to get behind the project.
“He supported the original concept when it was still considered impossible to do.”
That investment allowed them to achieve proof of principle. From there, they got money from Western Economic Diversification (WED) and more from the Cancer Foundation, to build the human size prototype. Building the clinical system was done with funds from ACF and Alberta Innovates. Alberta Health Services has provided significant in-kind support by covering the renovations and supporting staffing costs.
The full-scale clinical system being installed at the Cross Cancer Institute is almost ready for patients. The two remaining hurdles MagnetTx must clear in order to bring the Aurora-RT to market are, first, to demonstrate its clinical utility, by treating patients at the Cross, and second, to secure regulatory approval from the FDA, Health Canada, and CE Mark, so they can sell the system in North America and Europe.
Murray is driven by his rock-solid confidence that the Aurora-RT is a better way to treat cancer that will improve the lives of patients.
“It’s just a matter of plugging away and getting it done. Things take time and they take money. But knowing it’s not an abstract thing, we feel very confident that it will improve cancer treatment. In my mind, it’s pretty obvious it will do that if we achieve what we have set out to do. And I’m confident we’ll achieve what we set out to do.”


